Diseases, Disorders & Impairment of the Δ-6 Desaturase Pathway Causing Chronic Inflammation
- Diabetes (Type 1 & Type 2) including Neuropathy
- Lipid-Enveloped Viruses (including COVID series)
- Alzheimer’s
- Dermatological Conditions
- Cardiovascular Disease
- Inflammatory Bowel Disease
- Chronic Fatigue (including post viral syndromes)
- Fatty Liver Disease Including NAFLD
- Cancer
Special thanks to EFA / Eicosanoids Canadian specialist, Paul Beatty for his technical assistance.
INTRODUCTION:
Most physicians and healthcare professionals have not been exposed to EFA-based science. Although extremely important, the field is mischaracterized by many as simply an exploration of fish oil. It is so much more than that. Because the field of eicosanoid physiology and its strong link to pathophysiology of disease is technically complex, this article is limited to an overview focusing on impaired ∆-6 and a direct consequence of that impairment —decrease in production of PGE1. Compensating for this impaired pathway has not been fully exploited until now. This article’s purpose is to provide introductory insights — with the minimum of complexity — that most physicians have not yet seen.
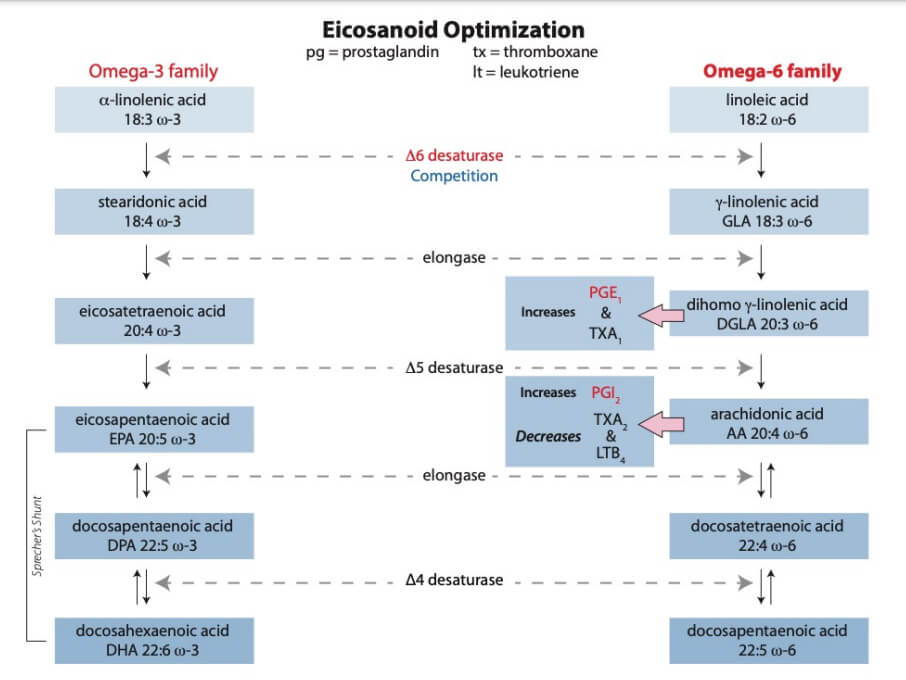
While treating patients for a particular disease / disorder, physicians may not be aware that the patient may also be suffering from an Essential Fatty Acid (EFA) impairment of the ∆-6 desaturase pathway (D6D). This impairment is increasing rapidly in the general population and in younger select patient populations. The increase in disease can be explained from a biologic, chemical, and pathophysiologic viewpoint. The body’s initial modulator of inflammation is PGE1, which requires CIS-LA (unadulterated Parent omega-6). This is accomplished via the ∆-6 Desaturase Pathway (See illustration – page 18).PGE1 is a potent systemic vasodilator, anti-inflammatory, and immune system regulator.
No modality of patient treatment will be optimized without understanding and optimizing patients’ EFA-based metabolic pathways; in particular, the significance of D6D and PGE1 optimization.
Importantly, PGE1 can exert both immunosuppressive and immunoenhancing activities in vivo — on an “as needed” basis by the body.1 PGE1 modulates critical T-cell activation with dysregulated release of interleukin (IL)-6, IL-17, and other cytokines.
Understanding a disease’s etiology is crucial. If the ∆-6 desaturase pathway is impaired or inhibited, all subsequent long-chain derivatives (GLA, DGLA, AA, EPA, DHA, etc.) — made via successive desaturate pathways — are also impaired. In addition to exacerbating disease states, this “cascade of impairment” would also be expected to impede wound (e.g., diabetic foot ulcer (DFU), and surgical healing, and it does.
“Auto-immune” diseases / disorders have become epidemic. A physiologic explanation is because an impaired ∆-6 desaturase pathway significantly decreases PGE1 output. PGE1 “throttles down” the inflammation cascade. [Technically, PGE1 raises level of cyclic AMP, 15(OH)DGLA – inhibits conversion of free
- 1. Winkelstein, A and Kelly, VE, “The Pharmacologic Effects of PGE1 on Murine Lymphocytes,” Blood (1980) 55 (3): 437-443.
Novel lipids-based pharmacognosy solutions
Treatment of diseases and disorders of impaired Δ-6 desaturase / inflammation
Arachidonic Acid to Leukotrienes & other metabolites of 5- and 12-lipoxygenases, and initiates T Lymphocyte Suppressor cells.]
Important note:
Pharmaceutical “anti-inflammatory” drugs (i.e., steroids / corticosteroids / NASIDs), work by “blocking or impeding” critical pathways (e.g., cyclooxygenase — COX-1 and COX-2, Lipoxygenase, etc.). Long-term, these drugs inhibit the ∆-6 Desaturase enzymatic pathway and decrease critical PGE1 and PGI2 signaling molecules — leading to serious side-effects. Short-term, “treat / minimize the symptoms” but inadvertently “feed the cause” — resulting in long-term negative effects.
To the contrary, optimizing PGE1 output via a properly calibrated EFA formulation, will NOT CAUSE harmful or unintended side-effects (e.g., addiction).*
* NSAIDs block the COX enzymes and reduce production of critically important prostaglandins. Steroids are even more disruptive to EFA metabolism, and long-term drug intolerance occurs. TNF inhibitors carry their own black box warnings.
As the following journal article details, there are a multitude of factors causing impairment of the ∆-6 desaturase pathway:2
“…Other factors which inhibit D6D activity are diabetes, alcohol and radiation, all of which may be associated with accelerated aging. PGE1 activates T lymphocytes, inhibits smooth muscle proliferation and thrombosis [blood clotting], is important in gonadal function and raises cyclic AMP levels in many tissues. It is a good candidate for a key factor lost in aging.”
An additional and often overlooked significant cause of impairment is because patients consume adulterated cooking oils; often over decades — laying the foundation for this metabolic impairment.3
Of course, an impairment in the functioning of one organ / tissue system, can lead to impairment of another organ / tissue system. For example, impairment of diabetic nerve functioning due to hypoxia — inducing reduced blood flow in the vascular system — leads to diabetic retinopathy, nephropathy, and increased cardiovascular distress. Chronic inflammation will start a chain reaction. If anti-inflammatory pathways are not activated, the results can be disastrous (e.g., See COVID / SARS below).
- 2. Horrobin, DF, “Loss of ∆-6 desaturase activity as a key factor in aging,” Medical Hypothesis. (1981) sep;7(9): 1211-20
- 3. Anton SD, et al., “Differential effects of adulterated versus unadulterated forms of linoleic acid on cardiovascular health,” J Integr Med, 2013; 11(1): 2–10.
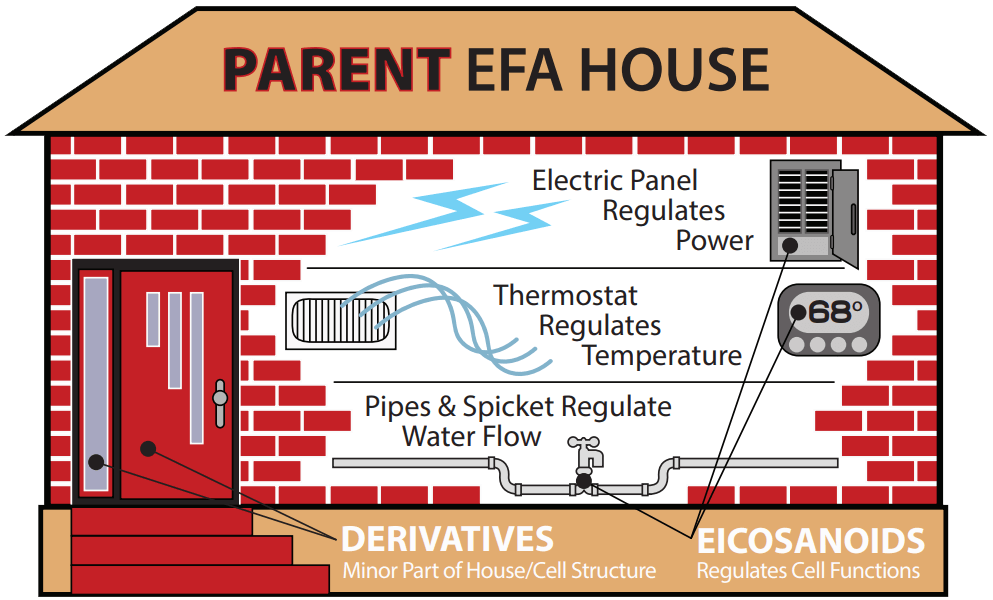
I find an illustration may be helpful. Picture a house in Houston, Texas, in late February 2021. The ambient temperature dropped dramatically even though the exterior structure was sound. Many people had to abandon their homes because utilities (electricity, heat, and water) were not properly functioning.
This house / utilities analogy is applicable to what is required from patients’ proper EFA metabolism. There are 3 main classes of EFA-related function:
- 1) Parents: The “brick and mortar” of each cell membrane (Parent omega-6 / -3; LA & ALA) — high quantities (25% – 33%) in of each of the body’s 100 trillion cell membranes.
- 2) Derivatives: Made from the 2 “Parents” in small quantities (e.g., GLA, DGLA, AA, EPA, DHA, etc.).
- 3) Eicosanoids: The complex signaling molecules. Made from the Derivatives in even smaller quantities. They may not even enter the bloodstream and can be extremely short-acting.
A home with broken / crumbling walls and great heat and water is insufficient for living, just as a home with great structure (lots of Parent EFAs only) and no electricity, heat, or water in winter is insufficient for living.
Note:
Quoted passages are used extensively. I want you seeing the scientist’s / researcher’s direct statements. Following is a brief, significantly referenced summary of diseases and disorders caused / associated with ∆-6 desaturase impairment:
Type I and Type II Diabetic Patients Suffer Impaired ∆-6 Desaturase
Diabetic patients often suffer from numerous issues such as retinopathy, neuropathy, nephropathy, and foot ulcers. It is well documented that diabetic patients have impaired ∆-6 desaturase (D6D) metabolic pathways from impaired insulin production.4,5,6 In particular, this metabolic defect causes a poor anti-inflammatory response in Type I patients. Even with insulin therapy, the D6D pathway is still deficient in Type I patients:7
“Diabetes, even when controlled by regular insulin injections, reduces the metabolism of linoleic acid, but the effect [of insulin use] is less than previously published. The fatty acid compositions of plasma and liver microsomal lipids are not reliable indices of the delta-6 desaturase activity in diabetes.”
Type II patients also suffer significant impairment of D6D activity.8 Compensating for this impaired pathway has not been fully addressed until now.
“Incubation of human erythrocyte membrane with low concentration of prostaglandin E1 [PGE1] or prostacyclin increased the binding of 125I-labeled insulin to the membrane…. The effect of prostaglandin E1 on the increased binding of the insulin was found to be reversible and depended on the occupancy of the autacoid molecules on the membrane and showed positive cooperativity…. Binding capacity increased 2- fold.”9
- 4. Brenner, RR, “Hormonal modulation of Δ-6 and Δ-5 desaturases: case of diabetes,” Prostaglandins, Leukotrienes, and Essential Fatty Acids, 68 (2003), 151-162.
- 5. Das, UN, “Essential fatty acids: biochemistry, physiology and pathology,” Biotechn., 2006, 1, 420-439.
- 6. Mikhailidis, DP, et al., “The effect of dihomogammalinolenic on platelet aggregation and prostaglandin release, erythrocyte membrane fatty acids and serum lipids: evidence for defects in PGE1 synthesis and Δ5-desaturase activity in insulin-dependent diabetics,” Diabetes Research (1986) 3,7-12.
- 7. Brown JE, Lindsay RM, Riemersma RA, “Linoleic acid metabolism in the spontaneously diabetic rat: Δ-6 desaturase activity vs. product/precursor ratios,” Lipids. 2000 Dec;35(12):1319-23.
- 8. Huang M, et al., “FADS Gene Polymorphisms, Fatty Acid Desaturase Activities, and HDL-C in Type 2 Diabetes,” Int. J. Environ. Res. Public Health, 2017, 14, 572.
- 9. Ray, TK, et al., “Regulation of insulin receptor activity of human erythrocyte membrane by prostaglandin E1 [PGE1],” Biochim Biophys Acta. 1986 Apr. 25,; 856(3):421-7;
Insulin sensitivity increases with PGE1 output.9
- Because of the reversibility of insulin binding, the impaired pathway must be compensated for on a daily basis.
“Levels of PGE1 in the serum of IDDs [insulin dependent diabetics] were significantly lower than those of the healthy volunteers p<0.002 at all sampling times.”10
“…These results demonstrated that PGE1 maintained the phenotype of VSMCs [vascular smooth muscle cells] via the AKT/mTOR-dependent autophagy, which prevented diabetes-induced vascular complications.11
Diabetic patients frequently consume processed foods containing adulterated oils.3 They are at great risk for chronic low-grade cellular inflammation — triggering long term cellular stress. The team, led by Professor Ernst, described how the UPR [unfolded protein response] senses nonfunctional / adulterated membrane lipids and responds accordingly, triggering chronic inflammation:12
“Cells in which the UPR [unfolded protein response] has been activated can produce larger quantities of proteins, but they also become more sensitive [hypersensitive] – a bit like a finely tuned race car. As Robert Ernst explains: ‘Whereas a racing car will often fail after completing a hundred super-fast laps because the engine has overheated, a tractor (representing a normal body cell) will continue to drive up and down the field for a lot longer, but also a lot slower.’ Why these high-performance cells are so much more sensitive was not known up until now.
“According to this new mechanism, the UPR is activated not only by misfolded proteins, but also by anomalous membrane lipid compositions. Secretory cells [e.g., the pancreas] are particularly sensitive to these changes, because they have already activated their UPR to produce more proteins and therefore at
- 10. Mikhailidis, DP, et al., “The effect of dihomogammalinolenic on platelet aggregation and prostaglandin release, erythrocyte membrane fatty acids and serum lipids: evidence for defects in PGE1 synthesis and Δ5-desaturase activity in insulin-dependent diabetics,” Diabetes Research (1986) 3,7-12; Dutta-Roy, A, “Effect of Evening Primrose Oil Feeding on Erythrocyte Membrane Properties in Diabetes Mellitus,” Omega-6 Essential Fatty Acids: Pathophysiology and Roles in Clinical Medicine, Wiley-Liss, NY, 1990, pages 505-511.
- 11. An X.-R., et al., “Prostaglandin E1 inhibited diabetes-induced phenotypic switching of vascular smooth muscle cells through activating autophagy,” Cell Physiol Biochem 2018;50:745–756.
- 12. Halbleib, K., et al., “Activation of the Unfolded Protein Response by Lipid Bilayer Stress,” Molecular Cell, Vol. 67, Issue 4, pp 673-684.e8, August 17, 2017; “Molecular biologists discover an active role of membrane lipids in health and disease,” August 4, 2017 in biology / cell & microbiology, phys.org.
risk of ‘overheating’ [inflamed] – just like the racing car engine described above. The study provides a new perspective on the active role of biological membranes may be a game changer for the understanding of a great variety diseases.
- “…[D]escribed how the UPR senses membrane lipids and responds accordingly. Working in close collaboration with scientists from Goethe University and the Max Planck Institute of Biophysics, both located in Frankfurt, the research team from the Department of Medical Biochemistry and Molecular Biology at Saarland University has identified a novel mechanism that leads to UPR activation and that can trigger long-term stress in cells [chronic inflammation].
- “In 2011, Peter Walter and David Ron, pioneer in the field of human UPR, came to regard the relationship between membrane lipids and the UPR as a central unresolved question in the field.
- “Both unfolded proteins and aberrant membrane lipid compositions3 [from consumed processed cooking oils] are sensed by Ire1… the UPR [unfolded protein response] senses membrane lipids and responds accordingly. According to this new mechanism, the UPR is activated not only by misfolded proteins, but also by anomalous membrane lipid compositions. Secretory cells are particularly sensitive to these changes….
“We now have the conceptual framework to understand why secretory cells [e.g., the pancreas] are hypersensitive to changes of their membrane lipids induced by the diet.”12
Diabetic Neuropathy (See separate whitepaper)
Diabetic Wound Healing (also See Cancer Section, pg. 15)
See / request the separate whitepaper devoted exclusively to expedited DFU healing. There was a fantastic article in Developmental Cell that apparently never made it into the clinical journals, nor did I include it in the DFU paper, so I’ll limit this section to its reference.
Fat cells, if fully functional, help to heal a wound; they can be motile and not exclusively stationary:13
“Fat body cells in Drosophila [a fly] play a surprising role [in their healing capability] of sealing wounds and preventing infection… The cells, which were previously thought to be immobile, propel themselves forward toward wounds with a wormlike wave motion, rather than adhering to and pushing off of other structures like most motile cells do.
- 13. Franz, A., et al., “Fat Body Cells Are Motile and Actively Migrate to Wounds to Drive Repair and Prevent Infection,” Developmental Cell 44, 460–470 (2018).
“After arriving at the site of a wound, fat body cells perform several useful functions [including minimizing infections]. “They work a lot harder and are more of a team player than was previously thought.… The fat cells crowd into the wound and waft debris to the edges of it, where the debris can be consumed by the immune cells. The fat cells are large enough that anywhere from one to four cells can plug the wound, playing a role similar to a clot or scab in vertebrates. The cells physically keep bacteria out of the wound while it heals, while helping increase the production of antimicrobial peptides to quell any infections. The fat cells stay at the wound site until it is healed. ‘Then they detach and just swim off, as though their job is done,’ Martin says.”
With processed / adulterated cooking oil consumption, their healing capability is impaired. Does this effect translate to humans? It likely does.
LIPID-ENVELOPED VIRUSES INCLUDING COVID / SARS
If your patient has an impairment in the ∆-6 desaturase pathway and contracts a virus, the effects could be devastating. The majority of viruses are enveloped with lipids. In particular, fatty acids of the omega-6 series are known to inactivate (lipid) enveloped viruses.14
“PUFAs have anti-bacterial, anti-viral, anti-fungal, anti-parasitic actions LA, ALA, and AA have bacteriostatic effect on both gram-positive and gram-negative bacteria. Both LA and AA can inactivate animal herpes, influenza, Sendai, and Sind-bis virus within minutes of contact.”15
“There are millions or billions of these viruses out there. The immune system fights back and attacks the virus; this is what causes inflammation and fever. But in extreme cases, the immune system goes berserk; particularly in the “Coronavirus” (SARS-CoV-2); [causing a cytokine explosion], causing more damage than the actual virus.”16 This harmful effect can be mitigated:
“Essential fatty acids are natural anti-inflammatory agents and therefore decrease the production of cytokines and histamine, which can contribute to neurotransmitter imbalance.”17
PGE1 activates (Cytotoxic) T Lymphocytes — the body’s powerful “killer cells” of intruders / infections / bacteria / viruses, etc. (British Society for Immunology).
- 14. Thormar, H., et al., “Inactivation of enveloped viruses and killing of cells by fatty acids and monoglycerides,” Antimicrob Agents Chemother. 1987 Jan; 31(1): 27–31; Yan, B, et al., “Characterization of the lipidomic profile of human Corona-virus infected cells: implications for lipid metabolism remodeling upon coronavirus replication,” Viruses 2019 Jan; 11(1): 73.
- 15. Das, UN, “Can essential fatty acids reduce the burden of disease(s),” Lipids In Health And Disease 2008, 7:9.
- 16. Yong, E., “Why the Coronavirus Has Been So Successful” Ed Yong, The Atlantic, Science Section (March 20, 2020).
- 17. “Essential Fatty Acids” chapter of the book, Integrative Medicine for Children, 2009.
An illustration of viral action will be used with COVID-19 / SARS since this type of virus causes fast-acting tissue / organ distress in patients with an existing ∆-6 desaturase impairment.
Research suggests a direct structural link between LA, COVID-19 pathology, and the virus itself and suggest that both the LA-binding pocket within the S protein and the multi-nodal LA (Parent omega-6) signaling axis, represent excellent therapeutic intervention points against SARS-CoV-2 infections.18
Acute respiratory distress syndrome (ARDS) can rapidly occur, causing severe shortness of breath as endothelial cells lining blood vessels and epithelial cells (Parent omega-6) lining airways lose their integrity, and protein rich fluid leaks into adjacent air sacs. For example, COVID-19 can cause insufficient oxygen levels (hypoxia) that has been seen in up to 80% of intensive care unit (ICU) patients exhibiting respiratory distress.19
In response to COVID-19 infection, both an immediate systemic innate immune response as well as a delayed adaptive response has been shown to occur. The virus can also cause a dysregulation of the immune response, particularly in the decreased production of T- lymphocytes. Severe cases tend to have lower lymphocyte counts, higher leukocyte counts and neutrophil-lymphocyte ratios, as well as lower percentages of monocytes, eosinophils, and basophils. Severe cases of COVID-19 show the greatest impairment in T-lymphocytes.
In the acute phase of COVID-19 infection, blood tests demonstrate elevated erythrocyte sedimentation rate (ESR), C-reactive protein, and other elevated inflammatory markers, typical for an innate immune response.20 Rapid viral replication can cause death of epithelial and endothelial cells and result in leaky blood vessels and pro-inflammatory cytokine release.
In those with severe disease, an uncontrolled release of pro-inflammatory cytokines — a cytokine storm — can occur. Cytokine storms originate from an imbalance in T-cell activation with dysregulated release of interleukin (IL)-6, IL-17, and other cytokines.
PGE1 “puts the brake” on “cytokine storm” as detailed in the medical journal articles. Cytokines, proteins, peptides and proteoglycans that modulate the body’s immune response, are elevated in patients with mild-to-moderate disease severity.21
Massive oxidative damage to the lungs has been observed in areas of consolidation documented on lung radiographs and CT scans in patients with COVID-19. Because disseminated virus can attach itself to cells containing an ACE-2 (angiotensin-converting enzyme 2) receptor, the disease can spread and damage
- 18. Toelzer, C, et al., “Free fatty acid binding pocket in the locked structure of SARS-CoV-2 spike protein,” Science 10.1126/science.abd3255 (2020).
- 19. Gattinoni, LD, et al., “COVID-19 pneumonia: different respiratory treatments for different phenotypes”: Intensive Care Medicine, 46 (6): 1099-1102 (2020).
- 20. Zhou, FY, et al., “Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study,” The Lancet 395 (10229):1054-1062.
- 21. Upadhyay, JN, et al., “Role of inflammatory markers in corona virus disease (COVID-19) patients: a review,” Experimental Biology and Medicine, 245 (15): 1368-1375.
organs and soft tissues throughout the body, including the lungs, heart, intestines, kidneys, blood vessels, fat, testes, and ovaries, etc. The disease can increase systemic inflammation and induce a hypercoagulable state. Without anticoagulation, intravascular blood clots can be devastating. However:
PGE1 increases blood flow (vasodilation) and inhibits platelet aggregation.22,23
ALZHEIMER’S
The beta-amyloid hypothesis as the cause of Alzheimer’s has failed and pharmaceutical success in the area remains largely ineffective. Alzheimer’s is now known as a cardiovascular disease caused by cellular impairment in the (at least 40 million) capillaries in the brain (comprised of exclusively Parent omega-6). The following extraordinary medical journal article published in 1990 “hits the nail on the head”:24
- “The findings strongly indicate abnormalities in ∆-6 desaturation. Alteration in PUFA desaturation/elongation processes and resultant membrane abnormalities may play a key role in the pathogenesis of Alzheimer’s disease. Membrane phospholipids are not only actual membrane constituents, but also determine membrane function. …[T]he findings strongly indicate abnormalities of ∆-6 desaturase in Alzheimer’s disease. The decrease in 22:6 (n -3) further supports altered ∆-6 desaturase activities. Abnormalities in the destruction/elongation process [initiating with ∆-6 desaturase] of PUFA (polyunsaturated fatty acid) and resultant membrane dysfunction may play a key role in the pathogenesis of Alzheimer’s disease.”
“Evidence is fast accumulating which indicates that Alzheimer’s disease is a vascular disorder with neurodegenerative consequences rather than a neurodegenerative disorder with vascular consequences.”25
Furthermore, as the 2011 Nature Reviews: Neuroscience article makes clear:26 “Patients with Alzheimer’s disease or other dementia-causing diseases frequently show focal changes in brain microcirculation. These changes include the appearance of string vessels (collapsed and acellular membrane tubes), a reduction in capillary density, a rise in endothelial pinocytosis, a decrease in mitochondrial content, accumulation of
- 22. Hissen, W, et al., Effect of prostaglandin E1 on platelet aggregation in vitro and in hemorrhagic shock,” Microvascular Research,” Vol 1, Issue 4, October 1969, pages 374-378.
- 23. Weiss, C., et al., “Hemostasis and fibrinolysis in patients with intermittent claudication: effects of prostaglandin E1, Prostaglandins, Leukotrienes and Essential Fatty Acids, Nov. 2000; 63(5):271–277.
- 24. 4 Nakada, T, et al., “Membrane fatty acid composition shows a Δ-6 desaturase abnormality in Alzheimer’s disease, NeuroReport 1, 153-155 (1990).
- 25. de la Torre, J.C. and Stefano, G.B., “Evidence that Alzheimer’s disease is a microvascular disorder: the role of constitutive nitric oxide,” Brain Research Reviews, Vol. 34, Issue 3, 2000, pages 119-136.
- 26. Zlokovic, B., “Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders,” Nature Reviews: Neuroscience, Vol. 12, December 2011, pages 723-738).
collagen and perlecans in the basement membrane, loss of tight junctions and/or adherens junctions, and BBB [blood-brain barrier] breakdown with leakage of blood-borne molecules….Increased levels of VEGF [vascular endothelial growth factor], a hypoxia-inducible angiogenic factor, were found in the walls of intraparenchymal vessels, perivascular deposits, astrocytes and intrathecal space of patients with Alzheimer’s disease, and were consistent with the chronic cerebral hypoperfusion and hypoxia that were observed in these individuals.”
“In a series of 300 autopsy cases of AD, Kalaria and Ballard reported 98% CAA [cerebral amyloid angiopathy], 100% microvascular degeneration, 31% infarcts of all sizes, and 7% intracerebral hemorrhage, while Olichney, in a cohort of 248 autopsy cases of AD, revealed a total of 48% CVLs [cerebrovascular lesions], with 31% microinfarcts, 12.5% large infarcts, and 13.5% hemorrhages.”27
“All of these pathologies may disrupt the integrity of cerebral vessels and alter brain perfusion leading to neuronal injury and cognitive impairment. SVD [cerebral small vessel disease] affects small arteries and arterioles and refers to pathological changes similar to atherosclerosis that are termed small vessel arteriosclerosis / atherosclerosis, lipo- or fibrohyalinosis, or hypertensive arteriopathy.”27
Dermatology: Epithelial / Epidermis Tissue /Atopic Dermatitis
There is an epidemic of dermatologic diseases. An understanding of EFA metabolism is required to combat this rise in cases. “Atopic dermatitis (AD) is a chronic inflammatory skin disorder characterized by eczematous lesions, skin dryness, and severe itch. AD affects 15–30% of children and 2–10% of adults. Atopic dermatitis (AD) has been related to a deficiency of ∆-6 desaturase….”28
The skin (epidermis / epithelial tissue) is comprised essentially of Parent omega-6 (LA) and arachidonic acid (AA) – a long chain omega-6 derivative. It is known that many dermatologic patients suffer from an impairment in the ∆-6 desaturase pathway: “ [S]uggests that the elongation of 18:3n 6 [via ∆-6 desaturase] into 20:3n 6 and its oxygenation to prostaglandins of the E series and lipoxygenase products of the 3-series may play a beneficial role in the management of cutaneous inflammatory hyperproliferative disorders.”29 Furthermore, there is identification of a fatty acid ∆-6 desaturase deficiency in human skin fibroblasts:30 [Fibroblasts are in the dermis underneath the outer layer of skin. Fibroblasts also allow skin to generate connective tissue to recover from injury.]
- 27. Attems, Johannes and Jellinger, Kurt A., “The overlap between vascular disease and Alzheimer’s disease – lessons from pathology,” (Vascular risk factors and Alzheimer’s disease), BMC Medicine, 2014, 12:206, pages 1-12.
- 28. Simon,D, et al., “Gamma-Linolenic Acid Levels Correlate with Clinical Efficacy of Evening Primrose Oil in Patients with Atopic Dermatitis,” Adv Ther (2014) 31:180–188.
- 29. Ziboh, VA and Chapkin, RS, “Metabolism and function of skin lipids,” Prog. Lipid Res. Vol. 27, pp. 81-105, 1988.
- 30. Willard, DE, et al., “Identification of a fatty acid ∆-6 desaturase deficiency in human skin fibroblasts,” The Journal of Lipid Research, 42, 2001, pages 501-508.
“In the present study we have investigated PUFA utilization in skin fibroblasts cultured from a female patient with clinical evidence of an inherited abnormality in fatty acid metabolism. The patient had a history of serious medical problems since shortly after birth, and GLC analysis of her plasma fatty acid composition indicated a low level of 20:4n-6 and DHA. Polyunsaturated fatty acid (PUFA) utilization was investigated in skin fibroblasts cultured from a female patient with an inherited abnormality in lipid metabolism. These deficient human skin fibroblasts (DF) converted 85–95% less [1-14C] linoleic acid (18:2n-6) to arachidonic acid (20:4n-6) …. These results suggested that DF are deficient in Δ-6 desaturation. This was confirmed…. A skin biopsy was obtained at the Kennedy Krieger Institute (Johns Hopkins University, Baltimore, MD), and a cultured fibroblast cell line (deficient human skin fibroblasts, DF) was established. Studies with rat liver microsomes indicate that a single Δ-6 desaturase acts on both 18- and 24-carbon PUFA substrates, whereas similar studies with human malignant cell lines suggest that separate Δ-6 desaturases act on these substrates. …These results demonstrate that the DF cells have a major deficiency in Δ6 desaturation.”
Cardiovascular disease
The lining of arteries (intima) and capillaries are comprised entirely of Parent omega-6. If this omega-6 is adulterated / nonfunctional from patients consuming processed foods / cooking oils, then the arteries / capillaries / veins will suffer inflammation, resulting in initiation of plaque, occlusions, and disfunction.3
A 1997 study published in the journal Arteriosclerosis, Thrombosis, and Vascular Biology, reported “Cholesterol esters [cholesteryl esterized with EFAs; in particular, Parent omega-6] are the predominant lipid fraction in all plaque types…” It also stated that “Intimal [innermost arterial lining] macrophages contain substantial amounts of cholesterol esters, which are rich in PUFAs [in particular, Parent omega-6].”31
The brilliant pathologist, Vladimir Subbotin, demonstrated to me high resolution images that, the endothelium intima (inner lining of the artery), is actually multi-layer — up to 30 layers in an adult, significantly increasing the potential for CVD.32
Intimal hypoxia (lack of oxygen) is also an initiating cause of CVD and fully functional Parent omega6 significantly improves cellular oxygenation. Dr. Campbell’s article, “Abnormal fatty acid composition and impaired oxygen supply in cystic fibrosis patients,” demonstrates that the Parent omega-6’s oxygen in the cell membrane can (reversibly) disassociate (release) oxygen — at physiologic pressure — increasing the tissue cellular oxygenation. In addition to the bloodstream, oxygen can come from the cell membrane itself.33
Chronic low-grade inflammation plays a role in cardiac hypertrophy and heart failure.5,34 Both microcirculation and microcirculation of the heart are improved with strong anti-platelet aggregation
- 31. Felton CV, Crook D, et al., “Relation of plaque lipid composition and morphology to the stability of human aortic plaque,” Arteriosclerosis, Thrombosis, and Vascular Biology, 1997;17:1337–1345.
- 32. Subbotin, V., “Excessive intimal hyperplasia in human coronary arteries before intimal lipid depositions in the initiation of coronary atherosclerosis and constitutes a therapeutic target,” Drug Discovery Today, Vol. 21, No. 10, October 2016.
- 33. Campbell, IM, et al., “Abnormal fatty acid composition and impaired oxygen supply in cystic fibrosis patients,” Pediatrics 1976; 57: 480–486.
- 34. Libby P. “Inflammation in atherosclerosis.” Nature. 2002 Dec 19–26;420(6917):868–874.
effect of PGE1.35 Increased cellular oxygenation / decreased hypoxia also occurs with increased PGE1.36 Increased blood flow and cardiovascular support also occur.37 The activation of PPARα (See PPAR and Δ6 desaturation below) is known to attenuate or inhibit the production of mediators of vascular damage, lipotoxicity, inflammation, reactive oxygen species (ROS), endothelial dysfunction, angiogenesis and thrombosis….”38
A 2000 study reported on the effectiveness of PGE1. German physician and researcher Clause Weiss, MD, et al., stated, “In summary, infusion therapy with PGE1 in patients with peripheral arterial occlusive disease (PAOD) reduces thrombin formation. PGE1 may thus reduce fibrin (thrombosis) deposition involved in the pathogenesis of atherosclerosis.”39
PGE1 may help dissolve occlusions / thromboses.
“Microcirculation and macrocirculation of the heart are improved with the strong anti-platelet aggregation effect of PGE1.”40
PPAR (Peroxisome Proliferator-Activated Receptors) and Δ-6 desaturation
Since the Δ-6 desaturase gene is influenced by PPAR, I’ll briefly reference it. Peroxisome proliferator activated receptors (PPARs) are ligand-activated transcription factors that belong to the nuclear hormone receptor superfamily. PPARα is mainly expressed in the liver, where it activates fatty acid catabolism. PPARδ is expressed ubiquitously and is implicated in fatty acid oxidation and keratinocyte differentiation. PPARγ2 is expressed exclusively in adipose tissue and plays a pivotal role in adipocyte differentiation. PPARγ is involved in glucose metabolism through the improvement of insulin sensitivity and represents a potential therapeutic target of type 2 diabetes.
- 35. Ren, H-X, et al., “Effect of prostaglandin E1 in patients with advanced lung cancer treated with chemotherapy,” Int J Clin Exp Med 2018;11(3):2285-2291.
- 36. Guo S, DiPietro LA. “Factors affecting wound healing.” J Dent Res. 2010;89(3):219–229
- 37. Das UN. “A defect in the activity of Δ6 and D5 desaturases may be a factor in the initiation and progression of atherosclerosis.” Prostaglandins Leukot Essent Fatty Acids. 2007;76(5):251–268; “[O]mega-6 PUFAs also have powerful anti-inflamatory properties that counteract any proinflammatory activity,’ say the advisory authors. ‘It’s incorrect to view the omega-6 fatty acids as “proinflammatory.”’ Ref.: Farvid MS et al. “Dietary linoleic acid [LA/ parent omega-6 and risk of coronary heart disease: a systematic review and meta-analysis of prospective cohort studies.” Circulation. 2014;130:1568–1578; Terano T et al. “Effect of oral administration of highly purified eicosapentaenoic acid on platelet function , blood viscosity and red cell in healthy human subjects.” Atherosclerosis. 1983;46:321–331.
- 38. Savary, S, et al., “Fatty acids ¾ Induced lipotoxicity and inflammation,” Current Drug Metabolism, 2012, Vol. 13, No. 10, pages 1358-1370.
- 39. Weiss, C., et al., “Hemostasis and fibrinolysis in patients with intermittent claudication: effects of prostaglandin E1,” Prostaglandins, Leukotrienes and Essential Fatty Acids, Nov. 2000; 63(5):271–277; ; Lazaro, I, et al., “Linoleic Acid Status in Cell Membranes Inversely Relates to the Prevalence of Symptomatic Carotid Artery Disease,” Stroke. 2021;52:703–706.
- 40. Ren, H-X, et al., “Effect of prostaglandin E1 in patients with advanced lung cancer treated with chemotherapy,” Int J Clin Exp Med 2018;11(3):2285-2291.
“The Δ-6 desaturase gene is known to contain a peroxisome proliferator response element and is under PPAR transcriptional control and may be the point of transcriptional control for both receptors within the essential fatty acid pathways.”41
Inflammatory Bowel Diseases
The entire lining of the digestive tract is Parent omega-6. Functional integrity is crucial. “Leaky gut” is caused by defects in the cellular membranes. Furthermore, PPAR is highly expressed in the colon, where bacterially induced signals increase its expression via TLR.5 “The Δ-6 desaturase gene is known to contain a peroxisome proliferator response element and is under PPAR transcriptional control and may be the point of transcriptional control for both receptors within the essential fatty acid pathways.”40
“Results demonstrated that pretreatment with PGE1 decreased the adhesion between vascular endothelial cells and monocytes, reduced the expression of vascular cell adhesion molecule-1, intercellular adhesion molecule-1, and E-selectin in vascular endothelial cells.
“In addition, PGE1 suppressed TNF-induced NF-kappaB activation and production of reactive oxygen species. “We concluded that PGE1 suppressed the vascular inflammatory process….” 42
Chronic Fatigue Including Post Viral Syndromes / Myalgic Encephalomyelitis
Chronic fatigue and exhaustion are widespread. We know that Parent omega-6 (LA) is the prime substrate of cardiolipin in the inner layer of the mitochondria — the creators of cellular energy. Additionally, Δ-6 desaturation impairment is a factor of the fatigue / energy impairment: 43
“Evidence is put forward to suggest that myalgic encephalomyelitis, also known as chronic fatigue syndrome, may be associated with persistent viral infection. In turn, such infections are likely to impair the
- 41. Kawashima Y, Musoh K, Kozuka H: “Peroxisome proliferators enhance linoleic acid metabolism in rat liver. Increased biosynthesis of omega 6 polyunsaturated fatty acids.” J Biol Chem. 1990, 265: 9170-9175; Roberts, L, et al., “The contrasting roles of PPARδ and PPARγ in regulating the metabolic switch between oxidation and storage of fats in white adipose tissue,” Genone Biology 12, Article number: R75 (2011).
- 42. Fang, W, et al., “Effect of prostaglandin E1 [PGE1] on TNF-induced vascular inflammation in human umbilical vein endothelial cells,” Can J Physiol Pharmacol. 2010 May;88(5):576-83
- 43. Puri, BK, “Long-chain polyunsaturated fatty acids and the pathophysiology of myalgic encephalomyelitis (chronic fatigue syndrome),” J Clin Pathol 2007;60:122–124.
ability of the body to biosynthesise n-3 and n-6 long-chain polyunsaturated fatty acids by inhibiting the d-6 desaturation of the precursor essential fatty acids…
“…[V]iral infections can prevent the body from biosynthesizing long-chain polyunsaturated fatty acids. In turn, this impairs the biosynthesis of membrane phospholipid molecules in the brain, as longchain polyunsaturated fatty acids are key components at the Sn2 position of these molecules. This leads to a reduced incorporation of the polar head group choline in these molecules (at the Sn3 position). Hence, we should expect to see a raised level of free choline in the brain, which can be assessed using proton neurospectroscopy.
“This is indeed the finding from the first two systematic proton neurospectroscopy studies thus far published on myalgic encephalomyelitis or chronic fatigue syndrome — one by our group. In 1935, Stoesser reported that acute viral infections were associated with a reduction in the levels of long-chain polyunsaturated fatty acids.
“That the cause of this reduction was the ability of many viral species to inhibit the d-6 desaturation of the precursor essential fatty acids was discovered four decades later by Dunbar and Bayley.
“As a result of viral or other inhibition of Δ-6 desaturase, an inadequate supply of the long-chain polyunsaturated fatty acids is available for incorporation into the membrane phospholipid molecules.” [Note: Most viruses, including Covid / SARS are lipid-enveloped and inactivated by long-chain fatty acids.”44]
Fatty Liver Disease Including NAFLD
Fatty liver disease consists of a variety of pathological states ranging from simple buildup of fat in the liver (hepatic steatosis) to nonalcoholic steatohepatitis, cirrhosis, and ultimately liver failure. This disease has reached epidemic proportions with up to 30% of Americans having some level of NAFLD. Reductions in hepatic insulin sensitivity are documented.
- “NAFLD is a low-grade systemic inflammatory condition. Increased formation of pro-inflammatory cytokines and eicosanoids and / or reduced formation of anti-inflammatory cytokines and inflammation resolving bioactive lipids may participate in the pathobiology of NAFLD.
- “Thus, release and timely formation of anti-inflammatory bioactive lipids is necessary to prevent NAFLD and / or resolution of inflammation seen in NAFLD….
- 44. Horowitz, B. et al., “Inactivation of lipid-enveloped viruses in labile blood derivatives by unsaturated fatty acids,” Vox Sang. 1988;54(1):14-20.
“Nonalcoholic fatty liver disease (NAFLD) is associated with decreased levels of AA, EPA and DHA and their anti-inflammatory products PGE1, PGD2, LXs, resolvins and protections with a concomitant increase in pro-inflammatory cytokines, IL-6 and TNF-alpha and bioactive lipids PGE2, LTs and TXs. The low levels of AA, EPA and DHA can be a result of decreased activities of Δ6 and Δ5 desaturases.”45
“An important aspect is the pathological process that occurs in nonalcoholic fatty liver disease (NAFLD), a condition in which oxidative stress of nutritional origin (fat and carbohydrates overload), in association with obesity, produces a significant and drastic decrease in the activity of the Δ-5 and Δ-6 desaturase enzymes in the liver.”46
Cancer & Radiation Therapy
Radiation therapy is widely used for treatment and examination. However, depending on the irradiation dose used, acute injurious effects of radiation on the skin, such as erythema, epilation, desquamation, hyperpigmentation, and erosion — referred to as radiodermatitis — are common side effects. Chronic radiation-induced skin ulcers are often observed in the region of radiodermatitis. These are characterized by poor healing and high relapse rate and are generally intractable.
- “Misoprostol, a [injectable] prostaglandin (PG) E1 analogue, is one of the most effective radiation protectors of the PGs investigated to date: 47
- “Wound healing was significantly delayed because of X-irradiation, but PGE1 administration prior to irradiation led to a significantly shorter delay in wound healing compared with controls.”
“Decreasing delay in wound healing was correlated with concentration of PGE1 administrated.…Thus, PGE1-administration may potentially alleviate the radiation-induced skin injury.”48
- “It has been indicated that experiments in the utilization of EFA in cancer modulation exist regarding intake and effect on cell structure and biochemical interactions within the cell in the prevention of cancer development.”49
- 45. Das, U, “A defect in the activities of Δ6 and Δ5 desaturase and pro-resolution bioactive lipids in the pathobiology of non-alcoholic fatty liver disease,” World Journal of Diabetes, 2011 November 15:2(11).
- 46. Wahli, W. and Michalik, L, “PPARs at the crossroads of lipid signaling and inflammation,” Trends in Endocrinology and Metabolism, July 2012, Vol. 23, No. 7, 351-363.
- 47. Hanson, WR, et al., “Radiation protection of the murine intestine by misoprostol, a prostaglandin E1 analogue, given alone or with WR-2721, is stereospecific,” Prostaglandins Leukot Essent Fatty Acids. 1988 Jun;32(3):101-5.
- 48. Takikawa, M, et al., “Protective effect of prostaglandin E1 on radiation-induced proliferative inhibition and apoptosis in keratinocytes and healing of radiation-induced skin injury in rats,” J. Radiat. Res., 53, 385-394 (2012).
- 49. Willard, DE, et al., “Identification of a fatty acid Δ6 desaturase deficiency in human skin fibroblasts,” The Journal of Lipid Research, 42, 2001, pages 501-508.
“A lower level of D6D was seen in breast tumors compared to normal tissues.”50
“When cells were exposed to PUFAs prior to but not during or following gamma-irradiation, the radiation treatment was enhanced by GLA but not EPA or DHA.”30
“Chronic inflammation is a major causative factor in human malignances. Pro-inflammatory cytokines influence tumor microenvironment and promote cell growth and survival and angiogenesis such that tumor cell growth is facilitated.”51
“EFAs/PUFAs play a significant role in such diverse conditions due to their ability to modulate cell membrane fluidity, possess second messenger action, influence angiotensin converting and HMG-CoA reductase enzymes, serve as ligands for nuclear receptors PPARs (peroxisome proliferator-activated receptors)….52
“In addition, PPARα is known to inhibit tumor growth and angiogenesis, which seem to be mediated by direct and indirect antiangiogenic effects and by its anti-inflammatory activity. A possible mechanism for PPARα-mediated tumor growth inhibition may involve suppression of signaling by hypoxia-inducible factor 1α (HIF-1α), as shown in cancer cells.”53
“As a result of viral or other inhibition of Δ-6 desaturase, an inadequate supply of the long-chain polyunsaturated fatty acids is available for incorporation into the membrane phospholipid molecules.” [Note: Most viruses, including Covid / SARS are lipid-enveloped and inactivated by long-chain fatty acids.”44]
Effect of prostaglandin E1 in patients with advanced
lung cancer treated with chemotherapy:40
“Chemotherapy is a common treatment for advanced lung cancer. However, although chemotherapy kills tumor cells, it also causes damage to normal cells.
“Thrombosis formation is easier in cancer patients than in healthy people due to their hypercoagulable state and thrombin produced by tumor cell membrane. Patients with advanced lung cancer usually have disorder of coagulation; therefore, they are more likely to develop VTE [venous thromboembolism] with chemotherapy treatment. Our study showed that PGE1 significantly reduced the incidence of VTE during chemotherapy in patients with advanced lung cancer.
“PGE1 leads to relaxation of smooth muscle and inhibits platelet aggregation as well as atherosclerotic lipid plaque formation. Chronic hypoxia [low cellular oxygen] can also generate secondary
- 50. Conklin, KA, “Dietary polyunsaturated fatty acids: Impact on cancer chemotherapy and radiation,” Alternative Medicine Review, Volume 7, No. 1, 2002, pages 4-21.
- 51. Das, UN, “Can essential fatty acids reduce the burden of disease(s),” Lipids In Health And Disease 2008, 7:9.
- 52. Hanson, WR, et al., “Radiation protection of the murine intestine by misoprostol, a prostaglandin E1 analogue, given alone or with WR-2721, is stereospecific,” Prostaglandins Leukot Essent Fatty Acids. 1988 Jun;32(3):101-5.
- 53. Echeverria, F, et al., “Long-chain polyunsaturated fatty acids regulation of PPARs, signaling: Relationship to tissue development and aging,” Prostaglandins, Leukotrienes and Essential Fatty Acids, 114 (2016)28-34.
polycythemia, increase blood viscosity and hematocrit, increase platelet adhesion effect of microcirculation perfusion, leading to thrombosis in pulmonary circulation and coronary circulation, and potential heart failure. PGE1 can dilate bronchial and artery vein blood vessels, and thus increase myocardial contractility. PGE1 inhibits the release of TXA2. Therefore, the TXA2 induced strong release and aggregation of platelet was inhibited and vasoconstrictive effect of platelet was reduced. This could contribute to the prevention of VTE….
- “In addition, PGE1 has a direct protective effect on vascular endothelial cells, which is beneficial to the production of tPA by endothelial cells and the enhancement of local fibrinolytic activity [preventing blood clots]….
- “Furthermore, chemotherapy will aggravate hyper-coagulable state and activation of blood coagulation facilitates cancer cells attachment, invasion and transfer which may in turn influence biology of the tumor, resulting in a poorer out-come of chemotherapy.
- “Patients treated with long-term chemotherapy may experience small pulmonary artery spasms, increase in pulmonary vascular resistance, pulmonary hypertension and increase of right heart load, which may lead to right heart failure or heart failure. Studies show that humoral factors such as prostaglandins [e.g., PGE1] play an important role in hypoxic pulmonary vasoconstriction.”