It is well documented that diabetic patients have impaired delta-6 desaturase (D6D) metabolic pathways from impaired insulin production.1,2,3 In particular, this metabolic defect causes a poor anti-inflammatory response in Type I patients. Even with insulin therapy, the pathway is still deficient.4 Type II patients also have significant impairment of D6D activity.5 Diabetic foot ulcer (DFU) wound healing is impaired.
This deficiency directly decreases PGE1 output. Both a powerful anti-inflammatory and vasodilator, PGE1 is critical to expedited DFU healing. Diabetic patients may possess only 42% of PGE1’s binding functionality — a 58% decrease compared with normal, non-diabetic patients.6 Steroids (glucocorticoids) further impair the Δ-6 desaturase pathway.1,7 During hypoglycemic episodes, the hormone glucagon is produced, further impeding the Δ-6 desaturase pathway (by means of cAMP).1,8
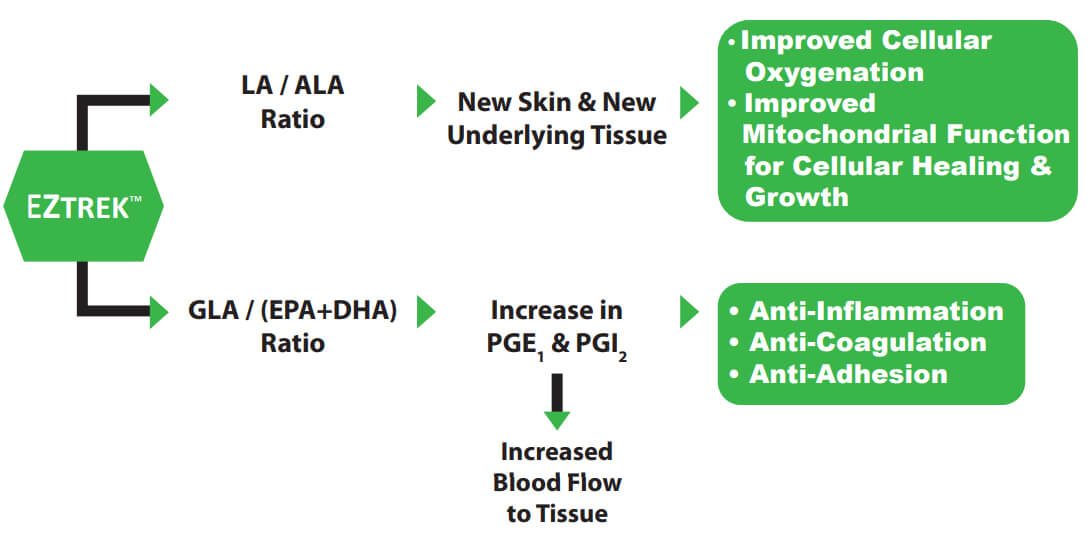
Compensating for impaired Δ-6 desaturase deficiency, the new medicament EZTREK™ —uniquely addresses underlying etiology — and simultaneously optimizes multiple metabolic pathways:
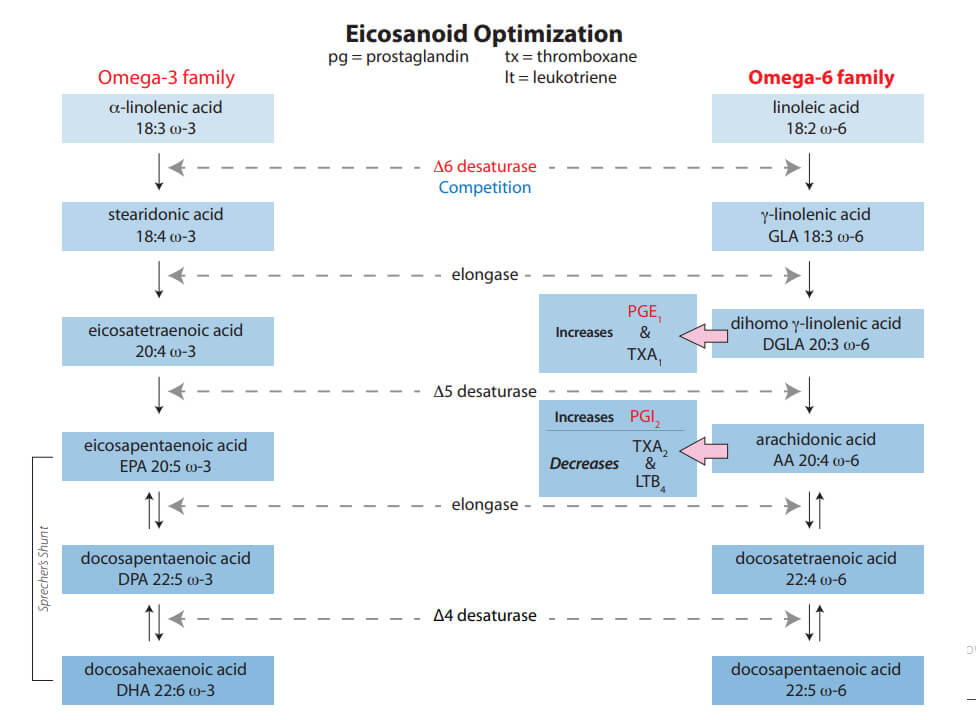
- The Δ-6 desaturase metabolic pathway favors the omega-3 series. Alpha-linolenic
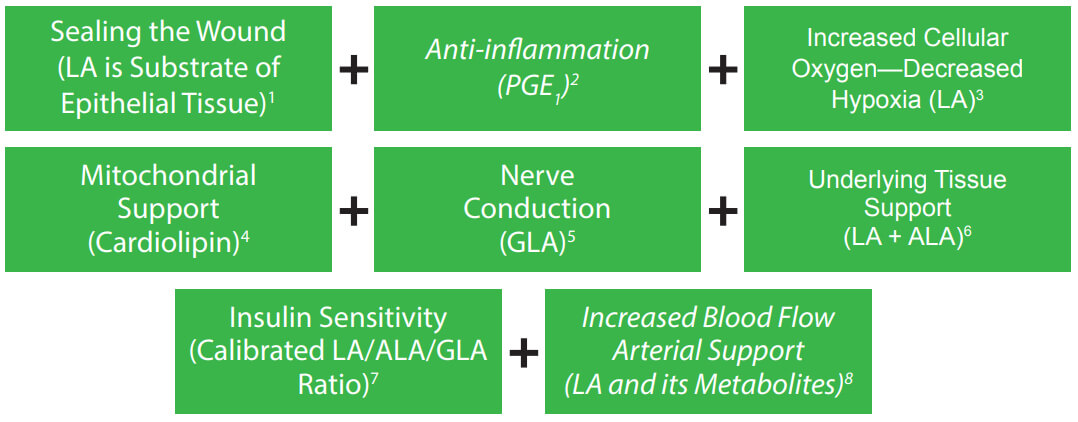
acid is important for tissue structure and support. However, PGE1 is produced exclusively from the omega-6 series. EZTREK™ solves this issue by specific calibration of both omega-6 / -3 series and with specific modulation of their long-chain metabolites.1,2,9,10
- EZTREK™ further enhances patients’ production of PGE1 by calibration of gamma-linolenic acid with docosahexaenoic acid.7,11
- Diabetic patients frequently consume (processed) foods that decrease the most fundamental substrate precursor of PGE1 —functional linoleic acid.1,2,12 Furthermore, the important cellular unfolded protein response (UPR) in secretory cells, such as the pancreas, is activated not only by unfolded proteins, but also by aberrant lipid composition (induced by the diet) of the ER membrane referred to as lipid bilayer stress. This response can trigger long-term stress (chronic inflammation) in cells.13 EZTREK™ calibrated EFA / eicosanoid modulating ratios are formulated to compensate for this and other obstacles that may impede the Δ-6 desaturase pathway.
- Fibroblasts in the dermis — important in allowing skin to regenerate connective tissue to recover from injury and maintain the extracellular matrix — are not maximized with Δ-6 desaturase deficiency.14
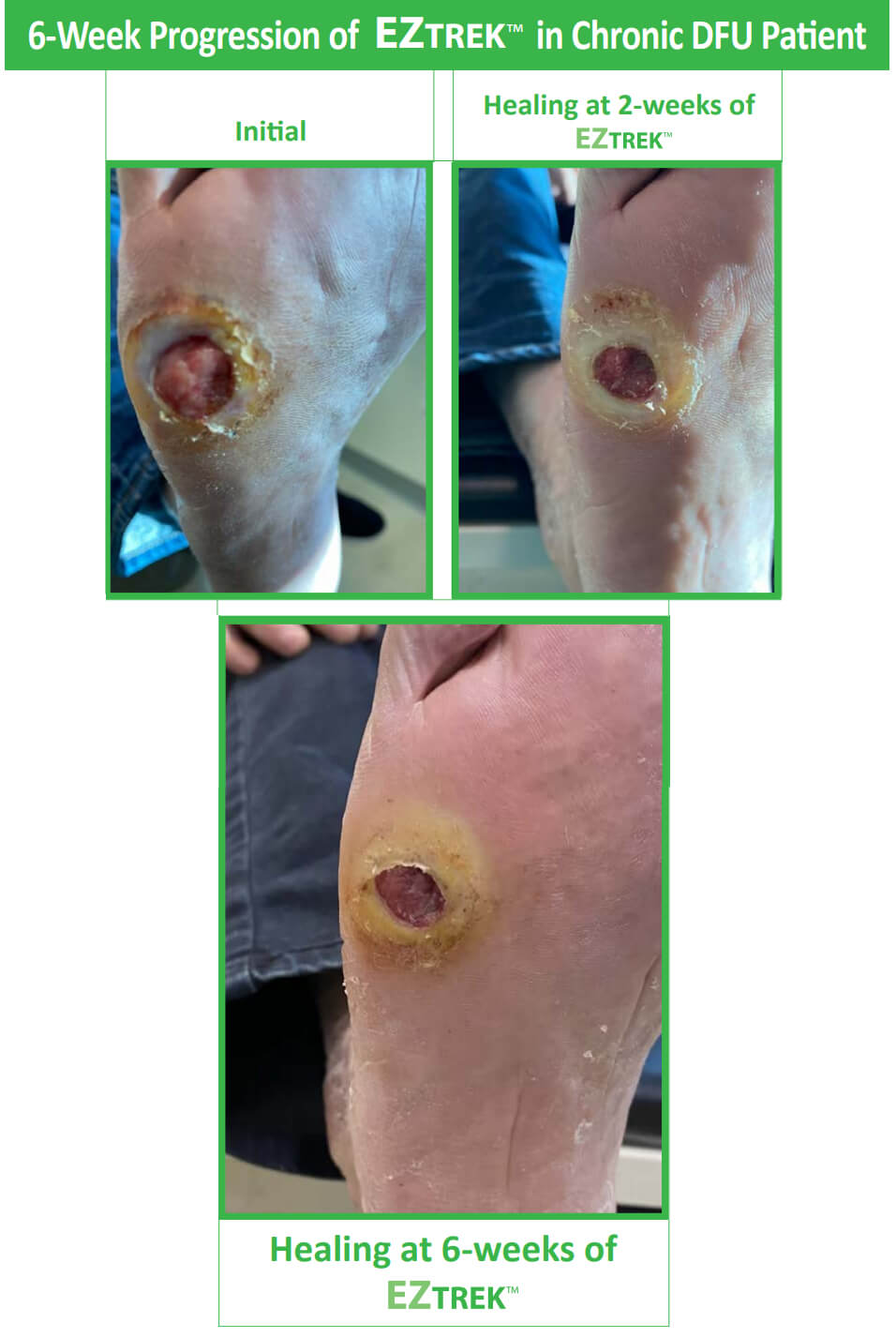
With continued EZTREK™ use, both acute and chronic DFUs heal faster.
- Brenner, RR, “Hormonal modulation of ∆-6 and ∆-5 desaturases: case of diabetes,” Prostaglandins, Leukotrienes, and Essential Fatty Acids, 68 (2003), 151-162.
- Das, UN, “Essential fatty acids: biochemistry, physiology and pathology,” Biotechn., 2006, 1, 420-439.
- Mikhailidis DP, et al., “The effect of dihomogammalinolenic acid on platelet aggregation and prostaglandin release, erythrocyte membrane fatty acids and serum lipids: Evidence and defects in PGE1 synthesis, and Δ5-desaturase activity in insulin-dependent diabetics,” Diabetes Research (1986), 3, 7-12.
- Brown JE, Lindsay RM, Riemersma RA, “Linoleic acid metabolism in the spontaneously diabetic rat: Δ-6 desaturase activity vs. product/precursor ratios,” Lipids. 2000 Dec;35(12):1319-23.
- Huang M, et al., “FADS Gene Polymorphisms, Fatty Acid Desaturase Activities, and HDL-C in Type 2 Diabetes,” Int. J. Environ. Res. Public Health, 2017, 14, 572.
- Dutta-Roy, Asim, “Effect of Evening Primrose Oil Feeding on Erythrocyte Membrane Properties in Diabetes Mellitus,” Omega-6 Essential Fatty Acids:
Pathophysiology and Roles in Clinical Medicine, Wiley-Liss, NY, 1990, pages 505-511.
- Brenner, RR, “Nutritional and hormonal factors influencing desaturase of essential fatty acids,” Prog Lipid Res., 1981;20:41-7.
- De Gomez Drumm, IT, de Alaniz, MT, Brenner, RR, “Effects of glucagon and dibutyryl adenosine 3’5’-cyclic monophosphate on oxidative desaturase of fatty acids in the rat,” J. Lipids Res., 16 (1975), 264-268.
- Brenner, RR, “Inhibitory effect of docosa-4,7,10,13,16,19-hexaenoic acid upon the oxidative desaturation of linoleic into gamma-linolenic acid and of alpha-linolenic into octadeca-6,9,12,15-tetraenoic acid,” Biochim. Biophys. Acta., 137 (1967), 184-186.
- Cho, HP, Nakamura, MT, Clarke, SD, “Cloning expression and regulation of human Δ-5 desaturase,” J. Biol. Chem, 274 (1999), 37335-37339. 11. Nassar, BA, et al., “The influence of dietary manipulation with n-3 and n-6 fatty acids on liver and plasma phospholipid fatty acidsin rats,” Lipids, 1986, Oct 21;10:652–656; Garg, ML, et al., “Δ-6 desaturase activity in liver microsomes of ratsfed enriched with cholesterol and / or ω3 fatty acids,” Biochem.J. (1988); 249:351–356.
- Anton, SD, et al., “Differential effects of adulterated versus unadulterated forms of linoleic acid on cardiovascular health,” J Integr Med, 2013; 11(1):2–10.
- Kristina Halbleib, et al., “Activation of the Unfolded Protein Response by Lipid Bilayer Stress,” Molecular Cell (2017); “Molecularbiologists discover an active role of membrane lipids in health and disease,” August 4, 2017 https://phys.org/news/2017-08-molecular-biologists-role-membrane-lipids.html
- Willard, DE, et al., “Identification of a fatty acid ∆-6 desaturase deficiency in human skin fibroblasts,” J. Lipids Res., 42, 2001, pages 501-508.